Research Group Translational Neurodegeneration
Overall aim of the group
Neurodegenerative diseases are age-related, i.e. their incidence rises continually with age. Due to the increasing life expectancy of the population, neurodegenerative diseases will constitute the largest group of age-related diseases in the future alongside with tumours and cardiovascular diseases. These diseases cannot be cured yet, and their progression can only be slowed down to a limited extent. Thus, neurodegenerative diseases represent an enormous socio-political and financial challenge for the time to come.
Neurodegenerative diseases are associated with amyloid-like protein aggregation, but the nature of the aggregates and their pathophysiological relevance have not yet been fully clarified. Both nuclear aggregation (e.g. in Huntington's disease) and cytoplasmic aggregation (Alzheimer's, ALS, Parkinson's) are known. This compartment specificity seems to play a crucial role in the pathophysiology, since aggregation in the cytoplasm leads to a disturbance of nucleocytoplasmic protein and RNA transport, while nuclear aggregation does not. These neurodegenerative mechanisms likely also define the selectivity and progression of the various neurodegenerative diseases.
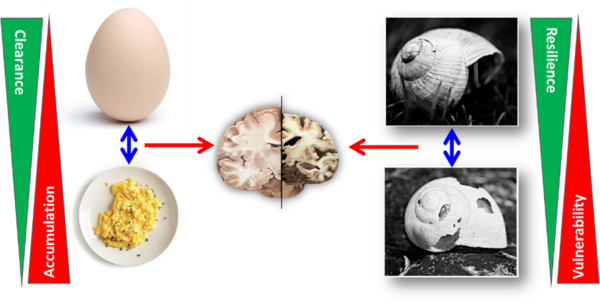
In addition, mechanisms of physiological and/or pathological aging play an important role in the pathophysiology of these diseases. Therefore, three complementary strategies for disease-modifying therapeutic intervention in neurodegenerative diseases are followed in our group:
- Supporting healthy ageing processes to strengthen physiological resilience (resistance to neurodegenerative insults)
- Reduction or deceleration of pathological aging processes
- Therapy of the neurodegenerative disease by intervention in its molceular pathophysiology
In fact, a combination of these three strategies offers an attractive solution for the therapy of neurodegeneration, particularly since many mechanisms that are currently believed to be (co-)responsible for neurodegeneration typically increase with age. Disease mechanisms and especially ageing processes are usually examined from the aspect of stressors or degenerative insults. The ability of the organism (or the brain) to resist these factors, i.e. resilience to neurodegenerative or age-related stressors, is attracting increasing attention in research.
Our group identified hypoexcitability in ALS-mutant motor neurons which could be rescued by 4-AP treatment (Naujock et al., Stem Cells. 2016) and we clinically translated it towards patient treatment (Peikert et al., J Clin Pharmacol 2019). We recently made significant progress in identifying DNA damage repair deficiency as a novel upstream event in ALS prior to and able to induce aggregation and neurodegeneration (e.g. Naumann et al., Nat Comm 2018, Kreiter et al. Neurobiol Dis 2018, Pal et al, Sci Data 2019). This, however, did not result in a general genotoxicity (e.g. tumor appearance) in ALS patients (Naumann et al., Ann Clin Transl Neurol. 2019). Additionally, we successfully unraveled the pathophysiological cascade of VPS13A/Chorea acanthocytosis (ChAc) by showing increased activity of Lyn kinase and showing a molecular link between active Lyn and impaired autophagy and that TKis Dasatinib or Nilotinib can rescue ChAc phenotypes in human ChAc derived cells and in Vps13a-/- mice(Stanslowsky et al. J Neurosci 2016, Lupo F et al., Blood 2016). We pioneered with a first in human individual off-label treatment approach with the FDA-approved TKi Dasatinib in three volunteer patients with ChAc. Dasatinib treatment (duration 25.8-50.4 weeks) was safe and well tolerated. The clinical outcomes remained stable and reduction of abnormally activated Lyn kinase and autophagy markers was found in erythrocytes (Peikert et al., J. Pers. Med. 2021).
Our future goal is to develop causal therapies of neurodegenerative diseases by combining above mentioned strategies (Figure). To achieve these goals, our group uses the entire spectrum of clinical research from fundamental to patient-oriented research. We combine long standing traditions of neuroscientific research at the Rostock University Medical Center by working on neurodegenerative diseases with up-to-date human stem cell-based cell systems. This mainly includes the use of patient-specific models and the development of individualized therapeutical strategies for neurodegenerative diseases in a bidircetional clinical translation approach.
Research Group Translational Neurodegeneration
Prof. Dr. Dr. Andreas Hermann (AG-Leiter)
Dr. Hannes Glaß (PostDoc)
Dr. Dajana Großmann (PostDoc)
Dr. Christiane Hartmann (PostDoc)
Dr. Marcel Naumann (Clinician Scientist)
Dr. Kevin Peikert (Clinician Scientist)
Dr. Alexandra Jürs (Clinician Scientist)
Dr. Muhammad Ismail (Medical Scientist)
Anna E. Bartalis (PhD Studentin)
Di Wu (PhD Student)
Annaliis Lehto (PhD Student)
Anze Karlek (PhD Student)
Leonarda Vucic (PhD Student)
Ece Eldem (PhD Student)
Constantin Minkner (MD Student)
Gael Nils Tarakdjian (MD Student)
Helene Block (MD Student)
Nina Malburg (MD Student)
Adrian Spranger (MD Student)
Christina Haß (MD Student)
Emily Fischer (MD Student)
Helene Block (MD Student)
Jona E. Brüning (MD Student)
My Dang Thi (MD Student)
Anna-Lena Fuchs (MD Student)
Lea Hellberg (MD Student)
Henri Maierhof (MD Student)
Maite Peters (MD Student)
Lorenz Reuter (MD Student)
David Sander (MD Student)
Jan Suchy (MD Student)
Jannes Wahl (MD Student)
Theresa Wierschin (MD Student)
Louisa Winkelmann (MD Student)
Video: Shown is the impact of microtubule disruption (24 hrs Nocodazole, 5µM, mid) or respiratory inhibition (24 hrs Oligomycin A, 10µM, bottom) exclusively at the distal site on the recruitment of WT FUS-GFP to the Laser cut in nuclei (boxed areas) at the proximal seeding site in microfluidic chambers. Note the unaltered FUS recruitment (proximal) despite the severe distal disruption of the mitochondria network along with loss of processive motility in the distally treated cells.

![[Translate to English:] -](/fileadmin/_processed_/c/c/csm_NEURO-060619108_fc893be133.jpg)




